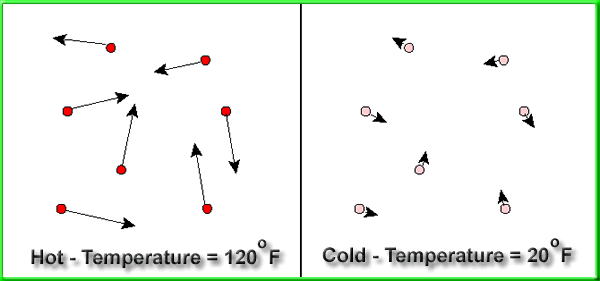
Above we see two boxes. The box on the left contains molecules
at a high temperature. These molecules have a high velocity,
a lot of KE, and therefore a lot of heat energy. The box
on the right contains molecules at a low temperature. These
molecules have a low velocity, low KE, and therefore little
heat energy.
These
two boxes illustrate a condition of low entropy because
they are very ordered. Most of the "hot" molecules
are on the left and most of the "cold" molecules
are on the right. There are not random. Remember, the more
randomness, the higher the entropy.
Because
the entropy is low, this energy is in a very useful state.
For example, we could use the "hot" molecules
to heat a room, since the temperature of 120 degrees F is
well above room temperature of around 70 degrees F. One
of the results of the second law of thermodynamics is that
heat energy always moves from the hot temperature to the
cold temperature. Likewise, we could use the "cold"
molecules to cool a room, since the temperature of 20 degrees
F is well below 70 degrees F.
What
happens after we use our energy to heat or cool our room?
It will be much the same as mixing the two boxes above.
When we are done, all of the molecules will have an average
temperature of around 70 degrees F as shown below.
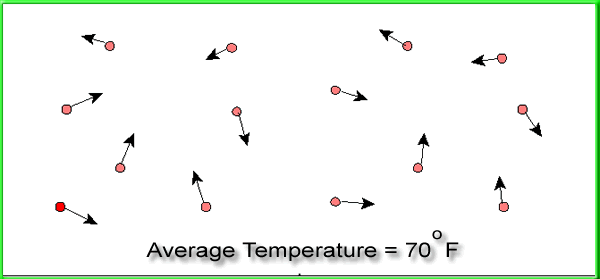
Now
the randomness has increased, since the molecules are not
longer separated into "hot" and "cold."
The entropy has therefore also increased. Not only that,
but the usefulness of the heat energy has decreased as well.
The molecules at 70 degrees F would not heat a room as well
as the molecules at 120 degrees F.
So,
the second law of thermodynamics basically says that the
universe MUST run downhill. The net result of any energy
changes must be an increase of entropy and the resulting
energy will be less useful and more difficult to use than
before. As energy changes form, as it does almost constantly,
it ages. This means that any energy resource must be finite,
and must run out if used often enough over a long enough
period of time.